Distinguish, differentiate, compare and explain what is the Difference between Isotopes and Isobars. Comparison and Differences.
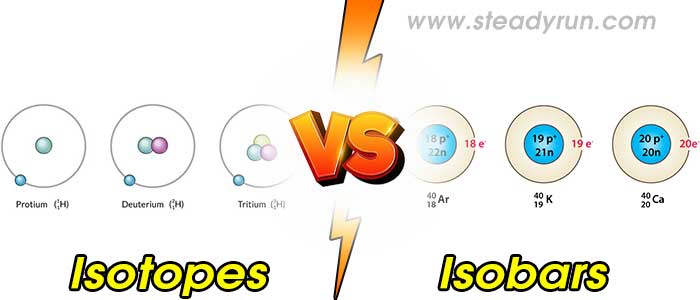
Isotopes are one series of nuclides have the same atomic number, but a different number of neutrons. Isotopes are atoms of the same element that have different mass numbers. They have the same number of protons, but different numbers of neutrons. They exhibit the same chemical properties. Examples: A carbon atom: It has 6 protons and 6 neutrons we call it "carbon-12" 12C6 because it has an atomic mass of 12. One useful isotope of carbon is "carbon-14", 14C6 which has 6 protons and 8 neutrons. Another example is the oxygen isotopes 15O8, 16O8, 17O8, 18O8.
Isobars are the atoms which have a different atomic number (the element is different) but the mass number is same. Example 14N7
Difference between Isotopes and Isobars
1. Isotope have the same atomic number. Isobars have a different atomic number.
2. Isotopes have a different mass number. Isobars have the same mass number.
3. In Isotopes, the number of protons is the same. In Isobars, the number of protons is different.
4. Isotopes occur at the same place in the periodic table. Isobars occur at a different place in the periodic table.
Tags:
Difference between Isobars vs Isotopes
Isotopes vs Isobars
Differences between Isobars vs Isotopes
Image Credits: Freepik








